- Top Results
- Bosch Sensortec Community
- Discuss
- MEMS sensors forum
- Re: BME 680 and BME 280 Not Reading Constant Humidity
BME 680 and BME 280 Not Reading Constant Humidity
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
BME 680 and BME 280 Not Reading Constant Humidity
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Report Inappropriate Content
11-23-2020 09:14 PM
I am using the BME 680 and the BME 280 in a sealed glass jar. I know the absolute humidity in the jar is constant because it is sealed.
However, when I get my data from the sensors and calculate the absolute humidty, I am not getting a contant absolute humdity. I have been getting data from the jar being indoors at room temperature and at atmospheric pressure.
Is this because of how the sensors work? Are they not made for monitoring a small, enclosed space? If it is because of how the sensors work, I need to know why this is happening so I can account for it in my calculations.
Thank you for your help!
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Report Inappropriate Content
11-24-2020 10:36 AM
Hi Sir:
Do you mean you can get correct humidity data at ambient temperature and at atmospheric pressure, and get incorrect humidity data at a small, enclosed space?
If yes, Incorrect data mean not to update humidity data or to get abnormal humidity data?
Other data, like pressure or temperature are normal?
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Report Inappropriate Content
11-24-2020 03:45 PM
Thank you so much for your response.
I am getting all accurate data except for seemingly, the relative humidity. It all looks right but when you calculate the absolute humidity from the temperature and relative humidity, it becomes inaccurate. (Because absolute humidity should be constant but the calculations don't show constant humidity)
I am wondering if it has to do with the programing of the relative humidity sensor.
It does not seem to be accurate for calculating the amount of water vapour in a closed container because it may not have been made to do so.
I am wondering if anyone has any information to help me understand why the sensor is giving me temperature and relative humidity data that do not correlate to constant humidity. I think it may have to do with how the relative humidity sensor does its calculations but I have no way to confirm this.
Thank you again
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Report Inappropriate Content
11-24-2020 04:38 PM - edited 11-24-2020 04:40 PM
Information about my calculations:
At first I used a formula I found online that has been used pretty widely:
Absolute Humidity = [6.122 * e^[17.67*T/(T+243.5)] * RH * 2.1674] / [273.15 + T]
I was seeing the errors I described. I do not get constant absolute humidity even though I know the conditions have absolute humidity.
I decided to take come up with my own method of calculating the absolute humidity:
1. I use the temperature data to calculate the Saturated Vapour Pressure (The partial pressure of water vapour when the air is saturated, before it would start to condense into liquid water):
ln(P2/P1) = -Hvap/R * [ (1/T2) - (1/T1) ]
Hvap: Heat of vapourization of water
R: Constant
T1 and P1: Temperature that water evaporates at at 1 atm. (373 K)
T2 and P2: Temperature from BME sensor and P2 is the saturated vapour pressure.
2. With the Saturated Vapour Pressure, I calculate the moles of water that would be present if the jar were actually saturated (n(saturated)) using the Ideal Gas Law.
n(saturated) = P2 * V / R * T
P2 and T: Calculated Saturated Vapour Pressure and the Temperature from the sensor
R: Constant
V: Volume of the Jar (1.7L)
3. With the moles at saturation and the Relative Humidity from the sensor, I can calulcate the actual number of moles (n(actual)) present in the jar.
RelativeHumidity = n(actual)/n(staurated) * 100%
With my new method of calculation, I am still seeing the same errors. The amount of water in the jar is not constant even though I know it should be. I thought the error may have been in the first formula, but now I know it is more likely to be an error within the sensors.
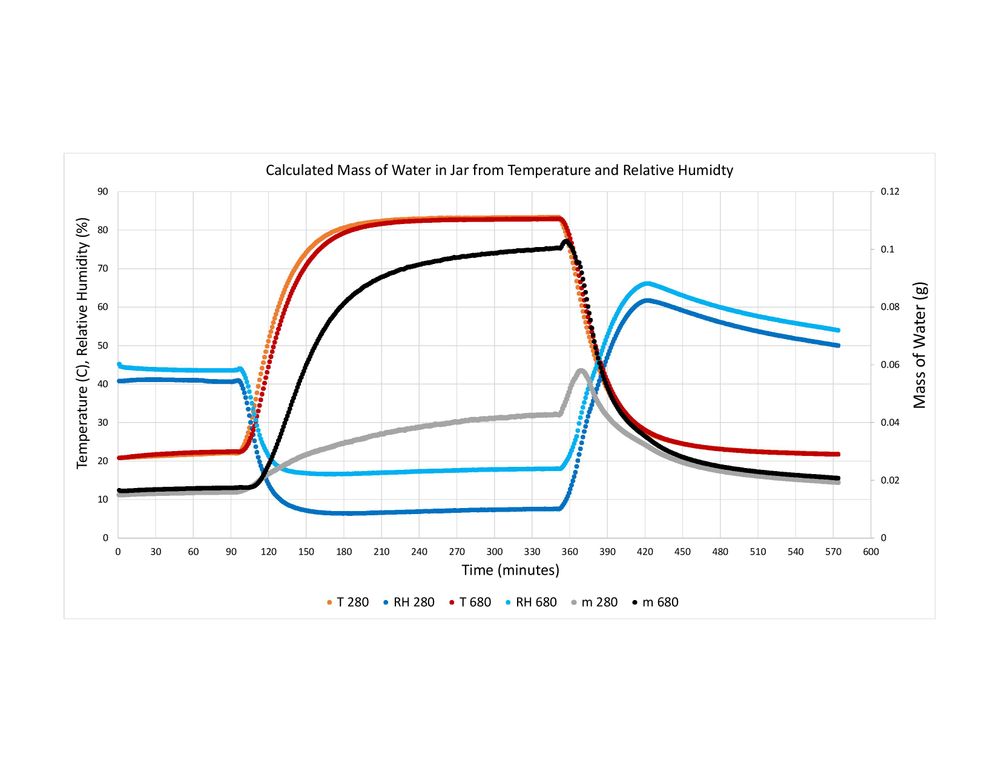
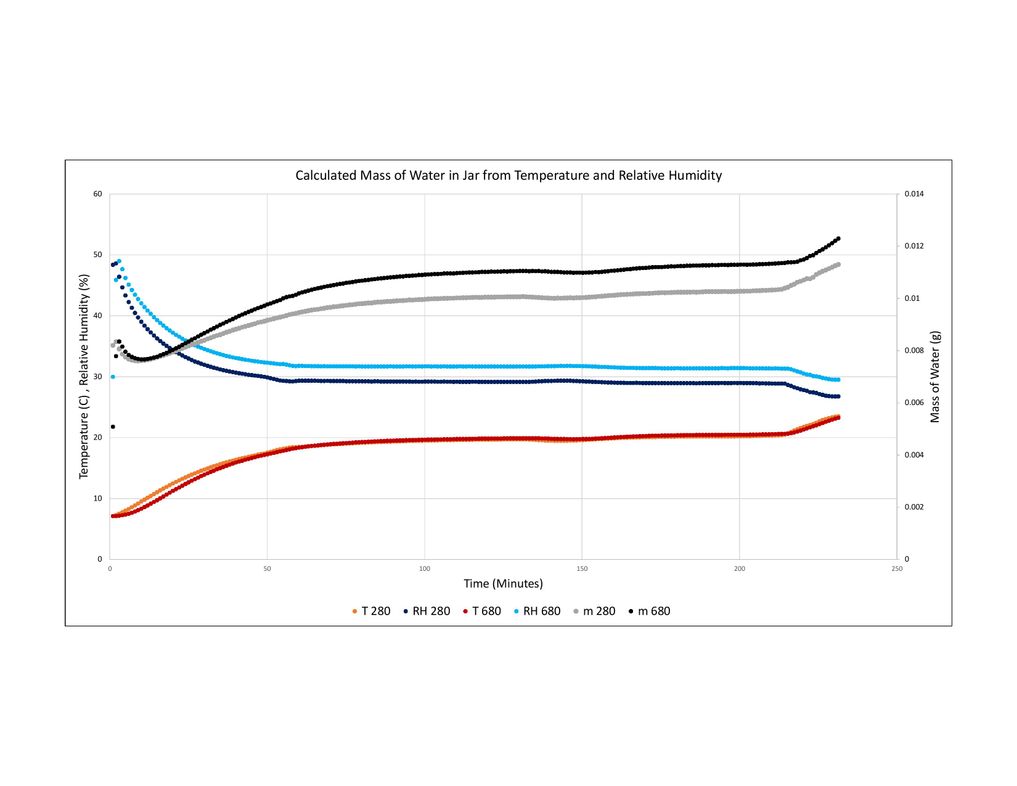
I have Included pictures of graphs I made from two of my tests. The data from the sensors is logged every 1 minute. The graphs show the Temperature and Relative Humidity read by the BME 280 and BME 680, and the water content that I calcluated using my new method. In one, the jar was put in an oven and brought back to room temperature. In the other, the jar is brough in from outisde and allowed to come up to room temperture.
Thank you for your help!
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Permalink
- Report Inappropriate Content
11-25-2020 07:56 AM - edited 11-25-2020 08:00 AM
Hi Sir:
Sorry, maybe I didn't understand clearly what you mean. Please do me a favor to tell me more information.
From your shown chart, these curve variations are basically fitted.
You mean the absolute humidity you calculate by the relaitve humidity and temperature of BME680 or BME280 is not in accord with the actual absolute humidity.
What is Your reference device or how do you know the actual absolute humidity ? and I don't see the actual absolute humidity value on the above picture.
I need more information to ask for internal experts' help if you wish.
Still looking for something?
- Top Results